Healthy skin is more than a cosmetic concern, it influences confidence, comfort, and overall well-being. Yet most acne and related treatments still depend on antibiotics, harsh chemicals, or invasive procedures that can irritate the skin and lose effectiveness over time.
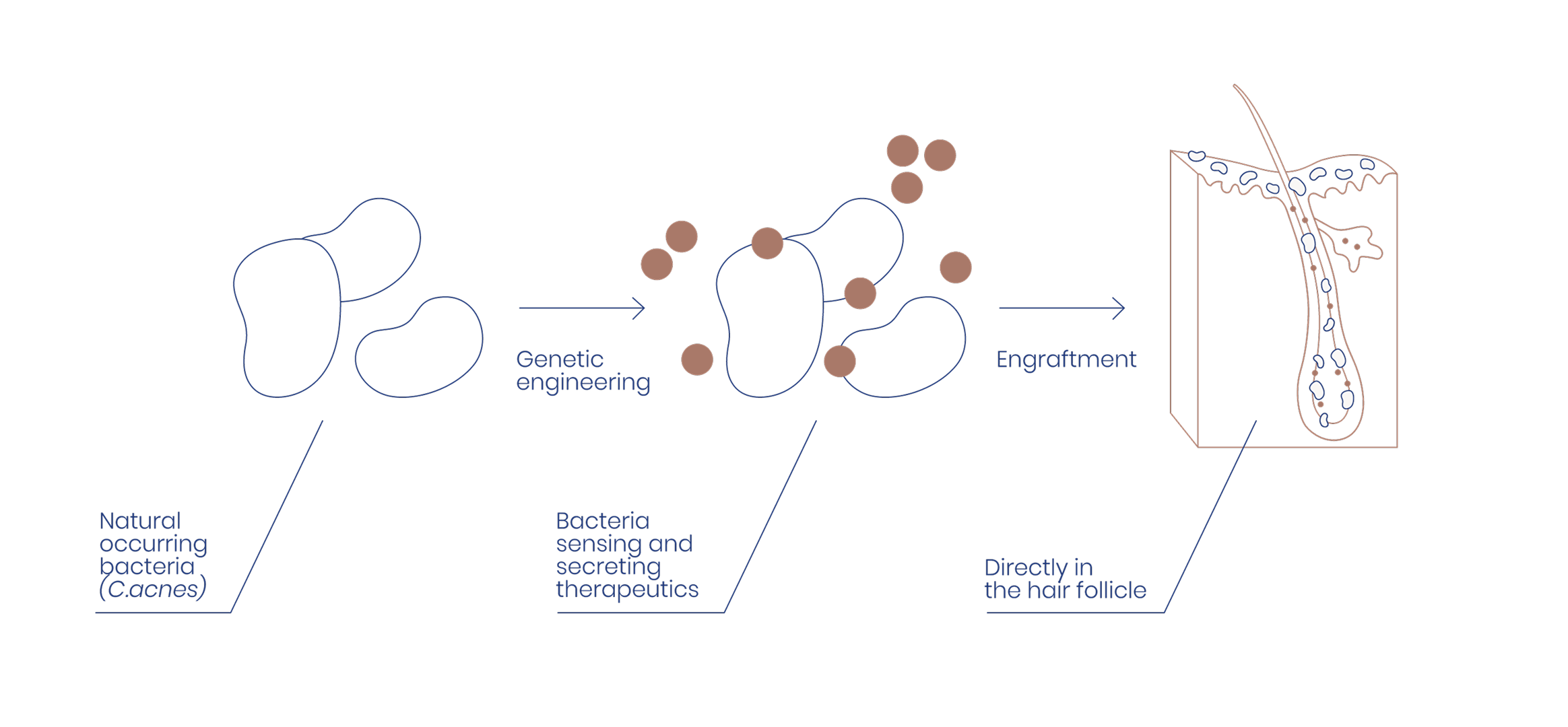
SkinEngineering introduces a new path: working with your skin’s natural microbiome to target oily skin and related conditions such as acne.
At the heart of SkinEngineering is the Synflora platform: a patented technology that engineers Cutibacterium acnes, one of the skin’s most common microbes, to deliver therapeutic molecules precisely where they are needed, inside the hair follicle. Our enhanced bacteria live naturally on the skin and continuously produce therapeutic molecules, offering a safe, non-invasive and long-lasting solution.
Our Three Pillars
Scientific validation through preclinical studies.
Upscaling & regulatory development.
Business & market strategy to accelerate clinical adoption.
Preclinical studies already demonstrate:
- Significant reductions in sebum and inflammation.
- Stable colonization in laboratory models.
With acne representing a €14 billion global market, SkinEngineering meets a clear need while also helping reduce antibiotic use, an important step in combating antimicrobial resistance.
Two complementary programmes, one pioneering platform
SkinEngineering is part of a broader vision powered by the Synflora platform.

1. SKINDEV Pathfinder grant (2023-2027): Using Synflora technology for atopic dermatitis with remarkable success.

2. SkinEngineering EIC Transition grant (2025-2028): Taking the platform further, applying it to acne and moving closer to clinical reality.

Together, these two lines of innovation show how one powerful microbial-engineering platform can transform skin care across different conditions, ushering in a future where our microbiome becomes a important factor in health.
Publications
Patents
1. WO2023139291A1 – Methods to produce recombinant Cutibacterium acnes and uses thereof
2. WO2023031342A1 – A method for screening for modifications in the infectivity range of bacteriophages due to epigenetic imprinting.
Other relevant publications
1. Paetzold, B.; Willis, J.R.; Pereira de Lima, J.; Knödlseder, N.; Brüggemann, H.; Quist, S.R.; Gabaldón, T.; Güell, M. (2019). Skin microbiome modulation induced by probiotic solutions. Microbiome Journal, 7: 95. doi: 10.1186/s40168-019-0704-2
2. Fábrega, M.J.; Knödlseder, N.; Nevot, G.; Sanvicente, M.; Toloza, L.; Santos-Moreno, J.; Güell, M. (2021). Establishing a Cell-Free Transcription–Translation Platform for Cutibacterium acnes to Prototype Engineered Metabolic and Synthetic Biology. ACS Biomaterials Science & Engineering. doi: 10.1021/acsbiomaterials.1c00894
3. Knödlseder, N.; Nevot, G.; Fábrega, M.J.; Mir-Pedrol, J.; Sanvicente-García, M.; Campamà-Sanz, N.; Paetzold, B.; Lood, R.; Güell, M. (2022). Engineering selectivity of Cutibacterium acnes phages by epigenetic imprinting. PLoS Pathogens, 18(3): e1010420. doi: 10.1371/journal.ppat.1010420
4. Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güell, M.; Paetzold, B. (2021). Skin microbiome transplantation and manipulation: Current state of the art. Computational and Structural Biotechnology Journal, 19: 624-631. doi:10.1016/j.csbj.2021.01.001.
5. Karoglan, A.; Pätzold, B.; Pereira de Lima, J.; Brüggemann, H.; Tüting, T.; Schanze, D.; Güell, M.; Gollnick, H. (2019). Safety and Efficacy of Topically Applied Selected Cutibacterium acnes Strains over Five Weeks in Patients with Acne Vulgaris: An Open-label, Pilot Study. Acta Dermato-Venereologica, 99(13): 1253–1257. doi:10.2340/00015555-3323.
6. Cros, M.P.; Mir-Pedrol, J.; Toloza, L.; Knödlseder, N.; Maruotti, J.; Zouboulis, C.C.; Güell, M.; Fábrega, M.J. (2021). New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders. Scientific Reports, 11: 20989. doi: 10.1038/s41598-021-00257-y.
7. Güell, M. (2023). Progress in sebaceous gland homeostasis, regeneration and immunomodulatory functions. Development, 150(15): dev202177. doi:10.1242/dev.202177.
8. Rozas, M.; Hart de Ruijter, A.; Fábrega, M. J.; Zorgani, A.; Güell, M.; Paetzold, B.; Brillet, F. (2021). From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium acnes to Skin Homeostasis. Microorganisms, 9(3): 628. doi:10.3390/microorganisms9030628.
9. Knödlseder, N. (2019). Why the bad is not always bad. Springer Nature Research Communities, published July 29, 2019.
10. Romaní, J.; Rosés, C.; Brillet, F.; Fernández-Vela, J.; Muñoz-Santos, C.; Fábrega, M. J.; Toloza, L.; Cros, M. P.; Güell, M.; Guilabert, A. (2025). Microbiome characterization of skin biopsies in Spanish patients with hidradenitis suppurativa shows a decrease in D1 and H1 lineages of Cutibacterium acnes. British Journal of Dermatology, 192(2): 359-361. doi:10.1093/bjd/ljae371
Funded by the European Innovation Council under Grant Agreement Number SkinEngineering 101203545.


And our future spin-off:

